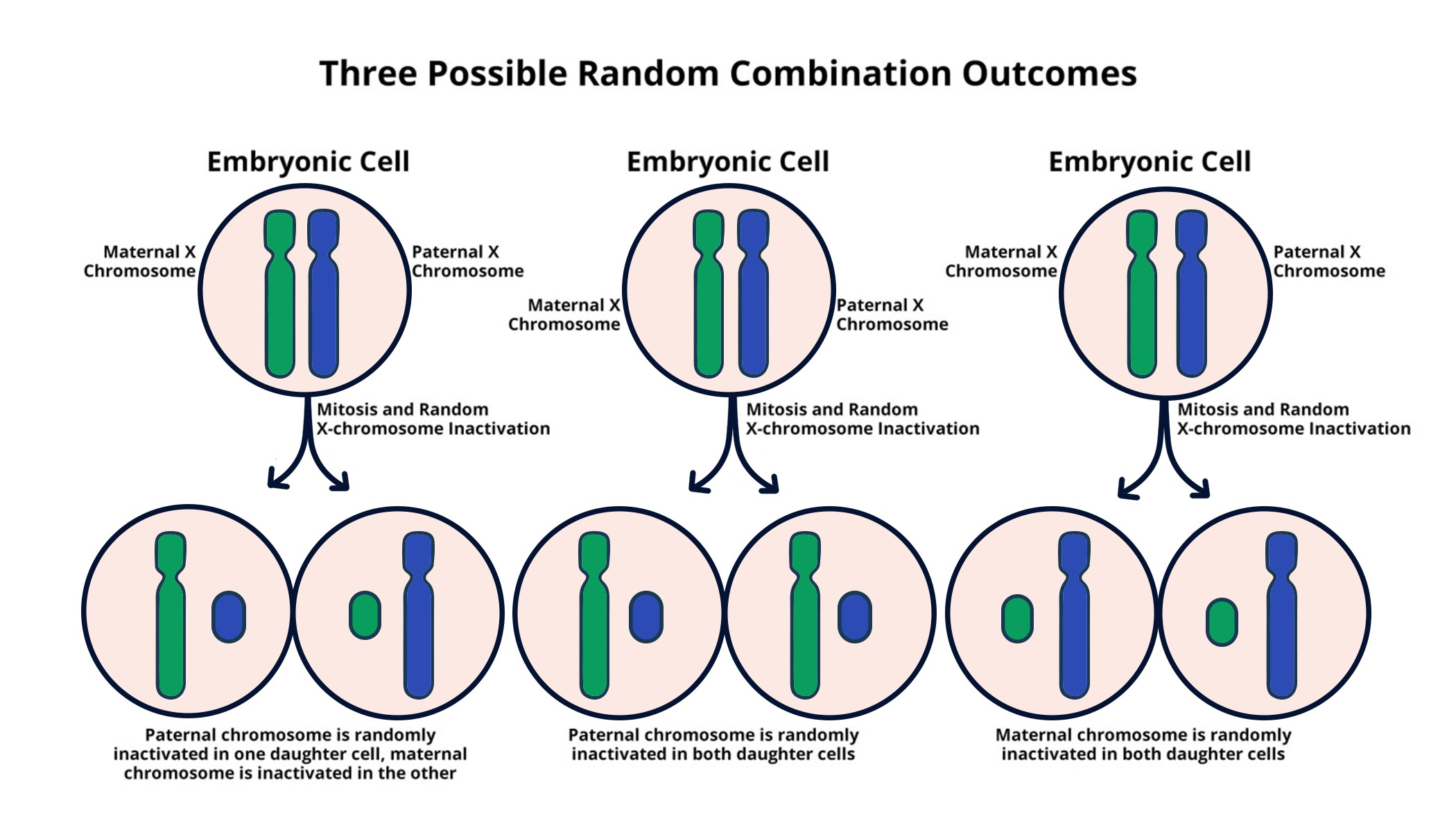
X chromosome inactivation is a fascinating and complex cellular process that plays a crucial role in balancing gene expression between sexes. In females, the presence of two X chromosomes presents a unique challenge, necessitating the silencing of one copy to maintain proper levels of X-linked gene products. This chromosomal silencing process has garnered significant attention, particularly in the context of X chromosome research, and is pivotal in understanding genetic disorders such as Fragile X Syndrome and Rett Syndrome. Recent advancements in gene therapy have opened new avenues for potential treatments, aimed at restoring normal function by targeting inactivated genes. With ongoing studies led by researchers like Jeannie Lee, there is hope that unraveling the mechanisms behind X chromosome inactivation could lead to groundbreaking therapies, fundamentally altering the landscape of genetic medicine for affected individuals.
The phenomenon known as X chromosome silencing is an intricate biological mechanism that regulates gene expression in females. While males have only one X chromosome, females possess two, prompting the need for one to become inactive through a sophisticated cellular strategy. This regulatory process is central to many areas of genetic inquiry, including the study of disorders like Fragile X Syndrome and Rett Syndrome. Current breakthroughs in chromosomal gene therapy aim to unlock these silenced genes, offering new hope for therapeutic applications. As researchers delve deeper into this chromosomal challenge, the potential for innovative treatments continues to expand, promising new avenues for those impacted by these genetic conditions.
Understanding X Chromosome Inactivation and Its Implications
X chromosome inactivation (XCI) is a fascinating process that allows female mammals to balance the gene dosage of X-linked genes. In females, with two X chromosomes, one X must be inactivated to equalize the expression levels with males, who possess a single X chromosome. This intricate chromosomal silencing process is orchestrated through the action of various molecules, notably the long non-coding RNA known as Xist. The role of Xist is critical as it initiates the inactivation by coating the X chromosome and transforming the surrounding chromatin structure into a more relaxed and accessible state, which profoundly impacts gene expression.
Recent research conducted by Jeannie T. Lee and her team has uncovered molecular insights into X chromosome inactivation, revealing its dependence on a gelatinous substance that behaves similarly to Jell-O. This unique chromosomal silencing not only plays a vital role in normal development but also offers therapeutic potential for a range of X-linked disorders. For instances like Fragile X Syndrome and Rett Syndrome, understanding XCI mechanisms allows researchers to devise strategies to unsilence mutated genes, potentially leading towards innovative treatments that could markedly improve patient outcomes.
Gene Therapy Advancements from X Chromosome Research
The advancements in gene therapy, particularly concerning X-linked disorders, have accelerated significantly over the past decades. With the foundational understanding of X chromosome inactivation, especially the mechanisms laid out by Lee’s research, scientists are now more equipped to tackle challenges posed by genetic mutations. A promising aspect of these advancements is the ability to target the inactivated X chromosome, restoring function to genes that are typically silenced due to mutations. This insight opens new avenues for therapeutic strategies that could alleviate conditions associated with intellectual disabilities and neurodevelopmental disorders.
As researchers continue to explore gene therapy avenues, they aim to refine techniques for safely reactivating genes on the inactivated X chromosome. Early-stage laboratory successes suggest that these approaches might not only benefit females—who rely on XCI to manage gene expression—but also males who may carry X-linked mutations. Innovations in gene therapy, driven by a deeper understanding of the chromosomal silencing process, hold great promise for the future of treatment options for conditions like Fragile X Syndrome, enhancing our potential for effective interventions.
The Role of Chromosomal Silencing in Disease Resolution
Exploring the role of chromosomal silencing, particularly within the X chromosome, proves essential for resolving prevalent genetic disorders. Recent findings demonstrate how certain mutations manifest clinically due to the inactivation of the corresponding healthy genes on the X chromosome. The ability to selectively unsilence these genes offers hope; the restoration of function could reverse the pathophysiological consequences associated with conditions like Fragile X Syndrome. Thus, understanding the chromosomal silencing process is not only an academic pursuit but also a clinical imperative.
As the research progresses, scientists are unraveling complexities behind why genes can be reactivated without impacting healthy gene pathways. These discoveries could lead to less invasive treatment strategies, minimizing potential side effects while maximizing therapeutic benefits. Moreover, the insight gained could inform future approaches to treating a broader spectrum of genetic disorders beyond those linked to the X chromosome, highlighting the expansive potential of gene therapy advancements rooted in breakthroughs in chromosomal research.
Exploring Fragile X Syndrome Treatment Strategies
Fragile X Syndrome (FXS) is a leading cause of inherited intellectual disability, and understanding its etiology has become paramount in developing effective treatment strategies. Advances in the research surrounding X chromosome inactivation have illuminated pathways through which these treatments can be delivered. By focusing on the mechanisms of XCI and employing gene therapy techniques that aim to reactivate the silenced genes on the X chromosome, treatments for FXS are inching closer to clinical application. This approach seeks to restore a healthy gene function that is often compromised due to mutations.
Future treatment strategies involve not only direct gene therapy but also enhancing the overall cellular environment to facilitate gene accessibility and expression. Researchers are exploring combinations of pharmaceutical and molecular techniques that can interact with the chromosomal silencing processes, offering comprehensive relief for those affected by FXS. The ongoing research promises not only to address the symptoms of Fragile X Syndrome but also to potentially alter the course of the disorder, granting affected individuals a better quality of life.
Understanding Rett Syndrome Therapy Approaches
Rett Syndrome, a neurodevelopmental disorder primarily affecting females, offers challenges similar to those faced in Fragile X Syndrome. The mechanisms of X chromosome inactivation provide valuable insights for therapeutic development in Rett Syndrome as well. Like FXS, Rett Syndrome also involves mutations on the X chromosome that can lead to a cascade of developmental issues. Therefore, understanding the dynamics of XCI may pave the way for innovative treatments that could reverse some of the effects caused by the mutated gene.
Current research is investigating how to manipulate the process of chromosomal silencing to reactivate genes that have become inactive due to mutations. Therapies aimed at addressing the symptoms of Rett Syndrome must consider individual genetic profiles to ensure efficacy. As advancements in gene therapy continue to evolve, they may provide the keys to not only treating Rett Syndrome but also improving the lives of individuals affected by various genetic disorders linked to the X chromosome.
The Future of Gene Therapy and Chromosomal Research
The landscape of genetic treatment is evolving rapidly, with gene therapy standing at the forefront due to ongoing studies related to chromosomal mechanisms. Understanding X chromosome inactivation has played a crucial role in developing innovative solutions for addressing genetic mutations that lead to various disorders. The future looks promising as researchers like Jeannie T. Lee lead efforts to turn basic research into tangible treatments. This shift from laboratory findings to clinical applications signifies a new era in genetic medicine.
In the coming years, we can anticipate an increase in clinical trials launching new therapeutic agents aimed specifically at X-linked disorders. The focus will revolve around safely unsilencing repressed genes, thereby restoring natural cellular functions. As methodologies become more refined and precise, the potential for curing genetically induced conditions grows, ultimately impacting countless lives worldwide. The collaborative nature of ongoing research, federal support, and advancements in technology will be instrumental in translating these discoveries into effective, real-world therapies.
Molecular Mechanisms Behind X Chromosome Inactivation
The molecular mechanisms that drive X chromosome inactivation are complex yet critical for understanding how gene expression is regulated in females. Research by Jeannie T. Lee and her colleagues has highlighted the role of Xist RNA in initiating the silencing process. This RNA interacts dynamically with chromatin, affecting how genes are expressed or repressed on the X chromosome. By delving deep into these molecular interactions, researchers are uncovering the intricate ways in which X-linked genes are controlled, paving the way for new therapeutic strategies.
In addition to Xist, several other factors contribute to this intricate process, including various chromatin-modifying enzymes and regulatory elements. The interplay between these molecules dictates the fate of the X chromosome and its potential for gene expression. Gaining insights into these processes not only expands the understanding of basic genetics but also aids in the exploration of therapeutic avenues for conditions linked to the X chromosome, offering hope for future treatments.
Clinical Applications from X Chromosome Research
Clinical applications stemming from X chromosome research have significant implications for treating various genetic disorders. With the groundwork laid out concerning X chromosome inactivation, scientists can explore targeted interventions to reactivate silenced genes, particularly those mutations known to cause disorders like Fragile X and Rett syndromes. This new understanding of gene regulation enables a shift towards innovative treatment modalities, which promise higher efficacy with fewer side effects compared to traditional therapies.
As research continues to produce promising results related to unsilencing genes within the inactivated X chromosome, the medical community is poised to make strides in transforming patient care for these complex conditions. The goal is to initiate clinical trials that could lead to safe and effective gene therapies, providing hope to many affected individuals and their families. Continued investment in this research area is vital for unlocking the potential for groundbreaking therapies that address the root causes of these debilitating disorders.
Challenges and Future Directions in X Chromosome Therapy
Despite the significant advancements in understanding X chromosome inactivation and associated treatments for genetic disorders, several challenges remain. The complexity of gene interactions on the X chromosome presents hurdles for developing effective therapies that can precisely target mutated genes without affecting healthy ones. Additionally, the potential for unintended consequences of reactivating genes must be thoroughly investigated, ensuring patient safety remains a priority.
Future research directions aim to refine the molecular techniques involved in gene therapy and expand our comprehension of the cellular environments that facilitate gene expression. As the scientific community grapples with the complexities of gene silencing and reactivation, collaborative efforts and innovative technology will be crucial for advancing therapeutic options for individuals suffering from conditions like Fragile X Syndrome and Rett Syndrome. Ultimately, perseverance in this research domain promises to unlock new treatment paradigms that could transform lives.
Frequently Asked Questions
What is X chromosome inactivation and how does it function in human cells?
X chromosome inactivation (XCI) is a vital process in female mammals, where one of the two X chromosomes is randomly silenced to prevent an overexpression of X-linked genes. This chromosomal silencing results from a gene called Xist, which plays a crucial role in modifying the chromosomal environment, allowing for effective inactivation. By understanding this process, researchers aim to explore potential treatments for genetic disorders like Fragile X Syndrome and Rett Syndrome.
How does X chromosome inactivation relate to therapeutic advancements for Fragile X Syndrome treatment?
Advancements in understanding X chromosome inactivation have significant implications for Fragile X Syndrome treatment. Research indicates that unsilencing the inactive X chromosome could restore the function of mutated genes linked to the disorder. By leveraging the chromosomal silencing mechanisms, therapies can be developed to activate healthy gene copies, thereby potentially alleviating symptoms of Fragile X Syndrome.
What role does the Xist gene play in the chromosomal silencing process during X chromosome inactivation?
The Xist gene is essential for the X chromosome inactivation process. It produces an RNA molecule that coats the X chromosome, altering the properties of the surrounding chromosomal environment, often described as a ‘Jell-O-like’ substance. This interaction facilitates the silencing of one X chromosome, ensuring that females regulate gene dosage effectively.
Can gene therapy advancements restore function to genes impacted by X chromosome inactivation?
Yes, recent gene therapy advancements suggest that it may be possible to restore function to genes impacted by X chromosome inactivation. By unsilencing the inactive X chromosome in individuals with conditions like Fragile X Syndrome and Rett Syndrome, researchers hope to enable cells to utilize healthy gene copies that were previously inaccessible due to inactivation.
What are the potential implications of chromosomal silencing processes for Rett Syndrome therapy?
The chromosomal silencing processes involved in X chromosome inactivation present exciting implications for Rett Syndrome therapy. As researchers develop methods to unsilence the inactive X chromosome, there is hope that individuals with Rett Syndrome may regain access to healthy copies of genes that can mitigate symptoms. This approach could lead to innovative treatments with minimal side effects.
How do scientific discoveries about X chromosome inactivation enhance our understanding of genetic disorders?
Scientific discoveries regarding X chromosome inactivation enhance our understanding of genetic disorders by revealing how certain genes are silenced and can potentially be ‘unsilenced.’ By understanding the mechanisms of XCI, researchers can explore targeted treatments for X-linked diseases like Fragile X Syndrome and Rett Syndrome, ultimately improving the clinical outcomes for affected individuals.
What are the challenges associated with freeing inactivated X chromosomes in research on genetic disorders?
Challenges in freeing inactivated X chromosomes include ensuring that therapeutic strategies effectively target mutated genes without affecting healthy ones. Scientists are working to understand why certain X-linked genes remain unaffected during treatment, aiming to optimize approaches to minimize side effects while maximizing therapeutic benefits for conditions such as Fragile X Syndrome and Rett Syndrome.
What research is being conducted to develop therapies based on X chromosome inactivation mechanisms?
Ongoing research, notably from Jeannie T. Lee’s lab, is focused on developing therapies that utilize the mechanisms underlying X chromosome inactivation. The lab is currently optimizing techniques to unsilence X-linked genes, with future plans to conduct safety studies and potentially move toward clinical trials aimed at treating Fragile X Syndrome and Rett Syndrome.
| Key Point | Details |
|---|---|
| X Chromosome Inactivation (XCI) | Females have two X chromosomes, while males have one. To balance gene expression and dosage between sexes, one X chromosome in females is inactivated. |
| Mechanism of Inactivation | Inactivation involves a substance likened to Jell-O, which surrounds chromosomes and helps separate them to avoid tangling. The RNA molecule Xist plays a crucial role by altering this substance around the X chromosome, leading to its silencing. |
| Potential Medical Applications | Understanding XCI can help in treating genetic disorders like Fragile X Syndrome and Rett Syndrome by potentially freeing inactivated X chromosomes, which could allow access to healthy genes. |
| Research Progress | Lee’s lab is developing techniques to unsilence genes from the inactive X, with hopes of initiating clinical trials for therapies in the coming years. |
| Challenges and Mysteries | While freeing mutated genes shows promise, it is unclear why healthy genes remain unaffected. There may be a limit to how many genes a cell can express at once. |
Summary
X chromosome inactivation is a crucial process in female mammals that equalizes gene expression between sexes by silencing one of the two X chromosomes. Recent research by Jeannie T. Lee and her team has uncovered the mechanisms of XCI, which could lead to innovative treatments for genetic disorders linked to the X chromosome. By manipulating the gelatinous material surrounding chromosomes and utilizing the RNA molecule Xist, significant advancements in therapy for conditions like Fragile X and Rett syndrome may soon be possible. This research not only highlights the complexities of genetic regulation but also opens doors to potential future treatments, emphasizing the importance of understanding X chromosome inactivation in medicine.